From Wikipedia
Open on Wikipedia
| Aphid Temporal range:
| |
|---|---|

| |
| The Oleander Aphid, Aphis nerii, a common aphid species found on plants in the milkweed family. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hemiptera |
| Suborder: | Sternorrhyncha |
| Infraorder: | Aphidomorpha |
| Superfamily: | Aphidoidea Latreille, 1802 |
| Families | |
| |
Aphids are small sap-sucking insects in the family Aphididae. Common names include greenfly and blackfly,[a] although individuals within a species can vary widely in color. The group includes the fluffy white woolly aphids. A typical life cycle involves flightless females giving live birth to female nymphs—who may also be already pregnant, an adaptation scientists call telescoping generations—without the involvement of males. Maturing rapidly, females breed profusely so that the number of these insects multiplies quickly. Winged females may develop later in the season, allowing the insects to colonize new plants. In temperate regions, a phase of sexual reproduction occurs in the autumn, with the insects often overwintering as eggs.
The life cycle of some species involves an alternation between two species of host plants, for example between an annual crop and a woody plant. Some species feed on only one type of plant, while others are generalists, colonizing many plant groups. About 5,000 species of aphid have been described, all included in the family Aphididae. Around 400 of these are found on food and fiber crops, and many are serious pests of agriculture and forestry, as well as an annoyance for gardeners. So-called dairying ants have a mutualistic relationship with aphids, tending them for their honeydew and protecting them from predators.
Aphids are among the most destructive insect pests on cultivated plants in temperate regions. In addition to weakening the plant by sucking sap, they act as vectors for plant viruses and disfigure ornamental plants with deposits of honeydew and the subsequent growth of sooty moulds. Because of their ability to rapidly increase in numbers by asexual reproduction and telescopic development, they are a highly successful group of organisms from an ecological standpoint.[1]
Large-scale control of aphids is not easy. Insecticides do not always produce reliable results, because of resistance to several classes of insecticide, and because aphids often feed on the undersides of leaves, and are thus shielded. On a small scale, water jets and soap sprays are quite effective. Natural enemies include predatory ladybugs, hoverfly larvae, parasitic wasps, aphid midge larvae, crab spiders, lacewing larvae, and entomopathogenic fungi. An integrated pest management strategy using biological pest control can work, but is difficult to achieve except in enclosed environments such as greenhouses.
Etymology
[edit]
The name aphid is from Carl Linnaeus's modern Latin, most likely from misreading the Middle Greek κόρῐς, koris, 'bug' as αφῐς, aphis.[2]
Distribution
[edit]Aphids are distributed worldwide, but are most common in temperate zones. In contrast to many taxa, aphid species diversity is much lower in the tropics than in the temperate zones.[3] They can migrate great distances, mainly through passive dispersal by winds. Winged aphids may also rise up in the day as high as 600 m where they are transported by strong winds.[4][5] For example, the currant-lettuce aphid, Nasonovia ribisnigri, is believed to have spread from New Zealand to Tasmania around 2004 through easterly winds.[6] Aphids have also been spread by human transportation of infested plant materials, making some species nearly cosmopolitan in their distribution.[7]
Evolution
[edit]

Fossil history
[edit]Aphids, and the closely related adelgids and phylloxerans, probably evolved from a common ancestor some 280 million years ago, in the Early Permian period.[9] They probably fed on plants like Cordaitales or Cycadophyta. With their soft bodies, aphids do not fossilize well, and the oldest known fossil is of the species Triassoaphis cubitus from the Triassic.[10] They do however sometimes get stuck in plant exudates which solidify into amber. In 1967, when Professor Ole Heie wrote his monograph Studies on Fossil Aphids, about sixty species had been described from the Triassic, Jurassic, Cretaceous and mostly the Tertiary periods, with Baltic amber contributing another forty species.[11] The total number of species was small, but increased considerably with the appearance of the angiosperms 160 million years ago, as this allowed aphids to specialise, the speciation of aphids going hand-in-hand with the diversification of flowering plants. The earliest aphids were probably polyphagous, with monophagy developing later.[12] It has been hypothesized that the ancestors of the Adelgidae lived on conifers while those of the Aphididae fed on the sap of Podocarpaceae or Araucariaceae that survived extinctions in the late Cretaceous. Organs like the cornicles did not appear until the Cretaceous period.[9][13] One study alternatively suggests that ancestral aphids may have lived on angiosperm bark and that feeding on leaves may be a derived trait. The Lachninae have long mouth parts that are suitable for living on bark and it has been suggested that the mid-Cretaceous ancestor fed on the bark of angiosperm trees, switching to leaves of conifer hosts in the late Cretaceous.[14] The Phylloxeridae may well be the oldest family still extant, but their fossil record is limited to the Lower Miocene Palaeophylloxera.[15]
Taxonomy
[edit]Late 20th-century reclassification within the Hemiptera reduced the old taxon "Homoptera" to two suborders: Sternorrhyncha (aphids, whiteflies, scales, psyllids, etc.) and Auchenorrhyncha (cicadas, leafhoppers, treehoppers, planthoppers, etc.) with the suborder Heteroptera containing a large group of insects known as the true bugs. The infraorder Aphidomorpha within the Sternorrhyncha varies with circumscription with several fossil groups being especially difficult to place but includes the Adelgoidea, the Aphidoidea and the Phylloxeroidea.[16] Some authors use a single superfamily Aphidoidea within which the Phylloxeridae and Adelgidae are also included while others have Aphidoidea with a sister superfamily Phylloxeroidea within which the Adelgidae and Phylloxeridae are placed.[17] Early 21st-century reclassifications substantially rearranged the families within Aphidoidea: some old families were reduced to subfamily rank (e.g., Eriosomatidae), and many old subfamilies were elevated to family rank. The most recent authoritative classifications have three superfamilies Adelgoidea, Phylloxeroidea and Aphidoidea. The Aphidoidea includes a single large family Aphididae that includes all the ~5000[3] extant species.[18]
Phylogeny
[edit]External
[edit]Aphids, adelgids, and phylloxerids are very closely related within the suborder Sternorrhyncha, the plant-sucking bugs. They are either placed in the insect superfamily Aphidoidea[19] or into the superfamily Phylloxeroidea which contains the family Adelgidae and the family Phylloxeridae.[12] Like aphids, phylloxera feed on the roots, leaves, and shoots of grape plants, but unlike aphids, do not produce honeydew or cornicle secretions.[20] Phylloxera (Daktulosphaira vitifoliae) are insects which caused the Great French Wine Blight that devastated European viticulture in the 19th century. Similarly, adelgids or woolly conifer aphids, also feed on plant phloem and are sometimes described as aphids, but are more properly classified as aphid-like insects, because they have no cauda or cornicles.[21]
The treatment of the groups especially concerning fossil groups varies greatly due to difficulties in resolving relationships. Most modern treatments include the three superfamilies, the Adelogidea, the Aphidoidea, and the Phylloxeroidea within the infraorder Aphidomorpha along with several fossil groups.[22]
| Sternorrhyncha |
| |||||||||||||||||||||||||||||||||
Internal
[edit]The phylogenetic tree, based on Papasotiropoulos 2013 and Kim 2011, with additions from Ortiz-Rivas and Martinez-Torres 2009, shows the internal phylogeny of the Aphididae.[23][24][25]
It has been suggested that the phylogeny of the aphid groups might be revealed by examining the phylogeny of their bacterial endosymbionts, especially the obligate endosymbiont Buchnera. The results depend on the assumption that the symbionts are strictly transmitted vertically through the generations. This assumption is well supported by the evidence, and several phylogenetic relationships have been suggested on the basis of endosymbiont studies.[26][27][28]
| Aphididae | |
Anatomy
[edit]
Most aphids have soft bodies, which may be green, black, brown, pink, or almost colorless. Aphids have antennae with two short, broad basal segments and up to four slender terminal segments. They have a pair of compound eyes, with an ocular tubercle behind and above each eye, made up of three lenses called triommatidia.[12] They feed on sap and plant fluids using piercing-sucking mouthparts called stylets, enclosed in a sheath called a rostrum, which is formed from modifications of the mandible and maxilla of the insect mouthparts.[29]
They have long, thin legs with two-jointed, two-clawed tarsi. The majority of aphids are wingless, but winged forms are produced at certain times of year in many species. Most aphids have a pair of cornicles (siphunculi), abdominal tubes on the dorsal surface of their fifth abdominal segment, through which they exude droplets of a quick-hardening defensive fluid[29] containing triacylglycerols, called cornicle wax. Other defensive compounds can also be produced by some species.[21] Aphids have a tail-like protrusion called a cauda above their rectal apertures.[12][30] They have lost their Malpighian tubules.[31]
When host plant quality becomes poor or conditions become crowded, some aphid species produce winged offspring (alates) that can disperse to other food sources. The mouthparts or eyes can be small or missing in some species and forms.[21]
Diet
[edit]
Many aphid species are monophagous (that is, they feed on only one plant species). Others, like the green peach aphid (Myzus persicae), feed on hundreds of plant species across many families. About 10% of species feed on different plants at different times of the year.[32]
A new host plant is chosen by a winged adult by using visual cues, followed by olfaction using the antennae; if the plant smells right, the next action is probing the surface upon landing. The stylus is inserted and saliva secreted, the sap is sampled, the xylem may be tasted and finally, the phloem is tested. Aphid saliva may inhibit phloem-sealing mechanisms and has pectinases that ease penetration.[33] Non-host plants can be rejected at any stage of the probe, but the transfer of viruses occurs early in the investigation process, at the time of the introduction of the saliva, so non-host plants can become infected.[32]
Aphids usually feed passively on sap of phloem vessels in plants, as do many other hemipterans such as scale insects and cicadas. Once a phloem vessel is punctured, the sap, which is under pressure, is forced into the aphid's food canal. Occasionally, aphids also ingest xylem sap, which is a more dilute diet than phloem sap as the concentrations of sugars and amino acids are 1% of those in the phloem.[34][35] Xylem sap is under negative hydrostatic pressure and requires active sucking, suggesting an important role in aphid physiology.[36] As xylem sap ingestion has been observed following a dehydration period, aphids are thought to consume xylem sap to replenish their water balance; the consumption of the dilute sap of xylem permitting aphids to rehydrate.[37] However, recent data showed aphids consume more xylem sap than expected and they notably do so when they are not dehydrated and when their fecundity decreases. This suggests aphids, and potentially, all the phloem-sap feeding species of the order Hemiptera, consume xylem sap for reasons other than replenishing water balance.[38] Although aphids passively take in phloem sap, which is under pressure, they can also draw fluid at negative or atmospheric pressure using the cibarial-pharyngeal pump mechanism present in their head.[39]
Xylem sap consumption may be related to osmoregulation.[38] High osmotic pressure in the stomach, caused by high sucrose concentration, can lead to water transfer from the hemolymph to the stomach, thus resulting in hyperosmotic stress and eventually to the death of the insect. Aphids avoid this fate by osmoregulating through several processes. Sucrose concentration is directly reduced by assimilating sucrose toward metabolism and by synthesizing oligosaccharides from several sucrose molecules, thus reducing the solute concentration and consequently the osmotic pressure.[40][41] Oligosaccharides are then excreted through honeydew, explaining its high sugar concentrations, which can then be used by other animals such as ants. Furthermore, water is transferred from the hindgut, where osmotic pressure has already been reduced, to the stomach to dilute stomach content.[42] Eventually, aphids consume xylem sap to dilute the stomach osmotic pressure.[38] All these processes function synergetically, and enable aphids to feed on high-sucrose-concentration plant sap, as well as to adapt to varying sucrose concentrations.[38]
Plant sap is an unbalanced diet for aphids, as it lacks essential amino acids, which aphids, like all animals, cannot synthesise, and possesses a high osmotic pressure due to its high sucrose concentration.[35][43] Essential amino acids are provided to aphids by bacterial endosymbionts, harboured in special cells, bacteriocytes.[44] These symbionts recycle glutamate, a metabolic waste of their host, into essential amino acids.[45][46]
Carotenoids and photoheterotrophy
[edit]Some species of aphids have acquired the ability to synthesise red carotenoids by horizontal gene transfer from fungi.[47] They are the only animals other than two-spotted spider mites and the oriental hornet with this capability.[48] Using their carotenoids, aphids may well be able to absorb solar energy and convert it to a form that their cells can use, ATP. This is the only known example of photoheterotrophy in animals. The carotene pigments in aphids form a layer close to the surface of the cuticle, ideally placed to absorb sunlight. The excited carotenoids seem to reduce NAD to NADH which is oxidized in the mitochondria for energy.[49]
Reproduction
[edit]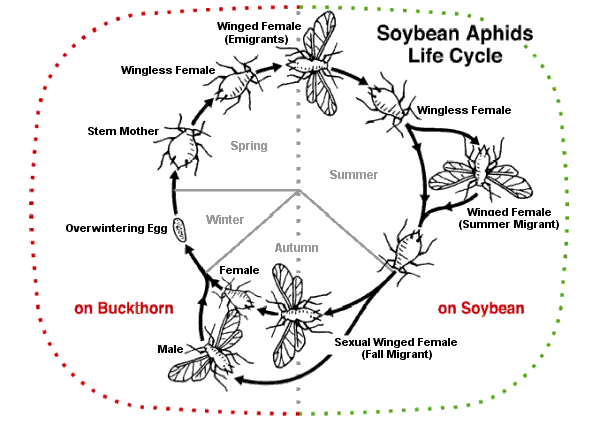
The simplest reproductive strategy is for an aphid to have a single host all year round. On this it may alternate between sexual and asexual generations (holocyclic) or alternatively, all young may be produced by parthenogenesis, eggs never being laid (anholocyclic). Some species can have both holocyclic and anholocyclic populations under different circumstances but no known aphid species reproduce solely by sexual means.[51] The alternation of sexual and asexual generations may have evolved repeatedly.[52]
However, aphid reproduction is often more complex than this and involves migration between different host plants. In about 10% of species, there is an alternation between woody (primary) hosts on which the aphids overwinter and herbaceous (secondary) host plants, where they reproduce abundantly in the summer.[21][51] A few species can produce a soldier caste, other species show extensive polyphenism under different environmental conditions and some can control the sex ratio of their offspring depending on external factors.[53]
When a typical sophisticated reproductive strategy is used, only females are present in the population at the beginning of the seasonal cycle (although a few species of aphids have been found to have both male and female sexes at this time). The overwintering eggs that hatch in the spring result in females, called fundatrices (stem mothers). Reproduction typically does not involve males (parthenogenesis) and results in a live birth (viviparity).[54] The live young are produced by pseudoplacental viviparity, which is the development of eggs, deficient in the yolk, the embryos fed by a tissue acting as a placenta. The young emerge from the mother soon after hatching.[55]
Eggs are parthenogenetically produced without meiosis[56][54] and the offspring are clonal to their mother, so they are all female (thelytoky).[12][55] The embryos develop within the mothers' ovarioles, which then give birth to live (already hatched) first-instar female nymphs. As the eggs begin to develop immediately after ovulation, an adult female can house developing female nymphs which already have parthenogenetically developing embryos inside them (i.e. they are born pregnant). This telescoping of generations enables aphids to increase in number with great rapidity. The offspring resemble their parent in every way except size. Thus, a female's diet can affect the body size and birth rate of more than two generations (daughters and granddaughters).[12][57][58]

This process repeats itself throughout the summer, producing multiple generations that typically live 20 to 40 days. For example, some species of cabbage aphids (like Brevicoryne brassicae) can produce up to 41 generations of females in a season. Thus, one female hatched in spring can theoretically produce billions of descendants, were they all to survive.[59]
In autumn, aphids reproduce sexually and lay eggs. Environmental factors such as a change in photoperiod and temperature, or perhaps a lower food quantity or quality, causes females to parthenogenetically produce sexual females and males.[56] The males are genetically identical to their mothers except that, with the aphids' X0 sex-determination system, they have one fewer sex chromosome.[56] These sexual aphids may lack wings or even mouthparts.[21] Sexual females and males mate, and females lay eggs that develop outside the mother. The eggs survive the winter and hatch into winged (alate) or wingless females the following spring. This occurs in, for example, the life cycle of the rose aphid (Macrosiphum rosae), which may be considered typical of the family. However, in warm environments, such as in the tropics or a greenhouse, aphids may go on reproducing asexually for many years.[29]

Aphids reproducing asexually by parthenogenesis can have genetically identical winged and non-winged female progeny. Control is complex; some aphids alternate during their life-cycles between genetic control (polymorphism) and environmental control (polyphenism) of production of winged or wingless forms.[60] Winged progeny tend to be produced more abundantly under unfavorable or stressful conditions. Some species produce winged progeny in response to low food quality or quantity. e.g. when a host plant is starting to senesce.[61] The winged females migrate to start new colonies on a new host plant. For example, the apple aphid (Aphis pomi), after producing many generations of wingless females gives rise to winged forms that fly to other branches or trees of its typical food plant.[62] Aphids that are attacked by ladybugs, lacewings, parasitoid wasps, or other predators can change the dynamics of their progeny production. When aphids are attacked by these predators, alarm pheromones, in particular beta-farnesene, are released from the cornicles. These alarm pheromones cause several behavioral modifications that, depending on the aphid species, can include walking away and dropping off the host plant. Additionally, alarm pheromone perception can induce the aphids to produce winged progeny that can leave the host plant in search of a safer feeding site.[63] Viral infections, which can be extremely harmful to aphids, can also lead to the production of winged offspring.[64] For example, Densovirus infection has a negative impact on rosy apple aphid (Dysaphis plantaginea) reproduction, but contributes to the development of aphids with wings, which can transmit the virus more easily to new host plants.[65] Additionally, symbiotic bacteria that live inside of the aphids can also alter aphid reproductive strategies based on the exposure to environmental stressors.[66]
In the autumn, host-alternating (heteroecious) aphid species produce a special winged generation that flies to different host plants for the sexual part of the life cycle. Flightless female and male sexual forms are produced and lay eggs.[67] Some species such as Aphis fabae (black bean aphid), Metopolophium dirhodum (rose-grain aphid), Myzus persicae (peach-potato aphid), and Rhopalosiphum padi (bird cherry-oat aphid) are serious pests. They overwinter on primary hosts on trees or bushes; in summer, they migrate to their secondary host on a herbaceous plant, often a crop, then the gynoparae return to the tree in autumn. Another example is the soybean aphid (Aphis glycines). As fall approaches, the soybean plants begin to senesce from the bottom upwards. The aphids are forced upwards and start to produce winged forms, first females and later males, which fly off to the primary host, buckthorn. Here they mate and overwinter as eggs.[50]
Ecology
[edit]Ant mutualism
[edit]Some species of ants farm aphids, protecting them on the plants where they are feeding, and consuming the honeydew the aphids release from the terminations of their alimentary canals. This is a mutualistic relationship, with these dairying ants milking the aphids by stroking them with their antennae.[b][68] Although mutualistic, the feeding behaviour of aphids is altered by ant attendance. Aphids attended by ants tend to increase the production of honeydew in smaller drops with a greater concentration of amino acids.[69]
Some farming ant species gather and store the aphid eggs in their nests over the winter. In the spring, the ants carry the newly hatched aphids back to the plants. Some species of dairying ants (such as the European yellow meadow ant, Lasius flavus)[70] manage large herds of aphids that feed on roots of plants in the ant colony. Queens leaving to start a new colony take an aphid egg to found a new herd of underground aphids in the new colony. These farming ants protect the aphids by fighting off aphid predators.[68] Some bees in coniferous forests collect aphid honeydew to make forest honey.[29]
An interesting variation in ant–aphid relationships involves lycaenid butterflies and Myrmica ants. For example, Niphanda fusca butterflies lay eggs on plants where ants tend herds of aphids. The eggs hatch as caterpillars which feed on the aphids. The ants do not defend the aphids from the caterpillars, since the caterpillars produce a pheromone which deceives the ants into treating them like ants, and carrying the caterpillars into their nest. Once there, the ants feed the caterpillars, which in return produce honeydew for the ants. When the caterpillars reach full size, they crawl to the colony entrance and form cocoons. After two weeks, the adult butterflies emerge and take flight. At this point, the ants attack the butterflies, but the butterflies have a sticky wool-like substance on their wings that disables the ants' jaws, allowing the butterflies to fly away without being harmed.[71]
Another ant-mimicking gall aphid, Paracletus cimiciformis (Eriosomatinae), has evolved a complex double strategy involving two morphs of the same clone and Tetramorium ants. Aphids of the round morph cause the ants to farm them, as with many other aphids. The flat morph aphids are aggressive mimics with a "wolf in sheep's clothing" strategy: they have hydrocarbons in their cuticle that mimic those of the ants, and the ants carry them into the brood chamber of the ants' nest and raise them like ant larvae. Once there, the flat morph aphids behave like predators, drinking the body fluids of ant larvae.[72]
-
An ant guards its aphids
-
Ants tending aphids
-
Ant extracting honeydew from an aphid
-
Ants tending aphids and collecting honeydew secreted. A wrinkled solder beetle flies in and eats an aphid before being chased away by the ants.
Bacterial endosymbiosis
[edit]Endosymbiosis with micro-organisms is common in insects, with more than 10% of insect species relying on intracellular bacteria for their development and survival.[73] Aphids harbour a vertically transmitted (from parent to its offspring) obligate symbiosis with Buchnera aphidicola, the primary symbiont, inside specialized cells, the bacteriocytes.[74] Five of the bacteria genes have been transferred to the aphid nucleus.[75] The original association is estimated to have occurred in a common ancestor 280 to 160 million years ago and enabled aphids to exploit a new ecological niche, feeding on phloem-sap of vascular plants. B. aphidicola provides its host with essential amino acids, which are present in low concentrations in plant sap.[76] The metabolites from endosymbionts are also excreted in honeydew.[77] The stable intracellular conditions, as well as the bottleneck effect experienced during the transmission of a few bacteria from the mother to each nymph, increase the probability of transmission of mutations and gene deletions.[78][79] As a result, the size of the B. aphidicola genome is greatly reduced, compared to its putative ancestor.[80] Despite the apparent loss of transcription factors in the reduced genome, gene expression is highly regulated, as shown by the ten-fold variation in expression levels between different genes under normal conditions.[81] Buchnera aphidicola gene transcription, although not well understood, is thought to be regulated by a small number of global transcriptional regulators and/or through nutrient supplies from the aphid host.[82]
Some aphid colonies also harbour secondary or facultative (optional extra) bacterial symbionts. These are vertically transmitted, and sometimes also horizontally (from one lineage to another and possibly from one species to another).[83][84] So far, the role of only some of the secondary symbionts has been described; Regiella insecticola plays a role in defining the host-plant range,[85][86] Hamiltonella defensa provides resistance to parasitoids but only when it is in turn infected by the bacteriophage APSE,[87][88] and Serratia symbiotica prevents the deleterious effects of heat.[89]
Predators
[edit]Aphids are eaten by many bird and insect predators. In a study on a farm in North Carolina, six species of passerine bird consumed nearly a million aphids per day between them, the top predators being the American goldfinch, with aphids forming 83% of its diet, and the vesper sparrow.[90] Insects that attack aphids include the adults and larvae of predatory ladybirds, hoverfly larvae, parasitic wasps, aphid midge larvae, "aphid lions" (the larvae of green lacewings), and arachnids such as spiders. Among ladybirds, Myzia oblongoguttata is a dietary specialist which only feeds on conifer aphids, whereas Adalia bipunctata and Coccinella septempunctata are generalists, feeding on large numbers of species. The eggs are laid in batches, each female laying several hundred. Female hoverflies lay several thousand eggs. The adults feed on pollen and nectar but the larvae feed voraciously on aphids; Eupeodes corollae adjusts the number of eggs laid to the size of the aphid colony.[91]
Aphids are often infected by bacteria, viruses, and fungi. They are affected by the weather, such as precipitation,[92] temperature[93] and wind.[94] Fungi that attack aphids include Neozygites fresenii, Entomophthora, Beauveria bassiana, Metarhizium anisopliae, and entomopathogenic fungi such as Lecanicillium lecanii. Aphids brush against the microscopic spores. These stick to the aphid, germinate, and penetrate the aphid's skin. The fungus grows in the aphid's hemolymph. After about three days, the aphid dies and the fungus releases more spores into the air. Infected aphids are covered with a woolly mass that progressively grows thicker until the aphid is obscured. Often, the visible fungus is not the one that killed the aphid, but a secondary infection.[92]
Aphids can be easily killed by unfavourable weather, such as late spring freezes.[95] Excessive heat kills the symbiotic bacteria that some aphids depend on, which makes the aphids infertile.[96] Rain prevents winged aphids from dispersing, and knocks aphids off plants and thus kills them from the impact or by starvation,[92][97][98] but cannot be relied on for aphid control.[99]
Anti-predator defences
[edit]
Most aphids have little protection from predators. Some species interact with plant tissues forming a gall, an abnormal swelling of plant tissue. Aphids can live inside the gall, which provides protection from predators and the elements. A number of galling aphid species are known to produce specialised "soldier" forms, sterile nymphs with defensive features which defend the gall from invasion.[29][100][101] For example, Alexander's horned aphids are a type of soldier aphid that has a hard exoskeleton and pincer-like mouthparts.[71]: 144 A woolly aphid, Colophina clematis, has first instar "soldier" nymphs that protect the aphid colony, killing larvae of ladybirds, hoverflies and the flower bug Anthocoris nemoralis by climbing on them and inserting their stylets.[102]
Although aphids cannot fly for most of their life cycle, they can escape predators and accidental ingestion by herbivores by dropping off the plant onto the ground.[103] Others species use the soil as a permanent protection, feeding on the vascular systems of roots and remaining underground all their lives. They are often attended by ants, for the honeydew they produce and are carried from plant to plant by the ants through their tunnels.[90]
Some species of aphid, known as "woolly aphids" (Eriosomatinae), excrete a "fluffy wax coating" for protection.[29] The cabbage aphid, Brevicoryne brassicae, sequesters secondary metabolites from its host, stores them and releases chemicals that produce a violent chemical reaction and strong mustard oil smell to repel predators.[104] Peptides produced by aphids, Thaumatins, are thought to provide them with resistance to some fungi.[105]
It was common at one time to suggest that the cornicles were the source of the honeydew, and this was even included in the Shorter Oxford English Dictionary[106] and the 2008 edition of the World Book Encyclopedia.[107] In fact, honeydew secretions are produced from the anus of the aphid,[108] while cornicles mostly produce defensive chemicals such as waxes. There also is evidence of cornicle wax attracting aphid predators in some cases.[109]
Some clones of Aphis craccivora are sufficiently toxic to the invasive and dominant predatory ladybird Harmonia axyridis to suppress it locally, favouring other ladybird species; the toxicity is in this case narrowly specific to the dominant predator species.[110]
Parasitoids
[edit]Aphids are abundant and widespread, and serve as hosts to a large number of parasitoids, many of them being very small (c. 0.1 inches (2.5 mm) long) parasitoid wasps.[111] One species, Aphis ruborum, for example, is host to at least 12 species of parasitoid wasps.[112] Parasitoids have been investigated intensively as biological control agents, and many are used commercially for this purpose.[113]
Plant-aphid interactions
[edit]
Plants mount local and systemic defenses against aphid attack. Young leaves in some plants contain chemicals that discourage attack while the older leaves have lost this resistance, while in other plant species, resistance is acquired by older tissues and the young shoots are vulnerable. Volatile products from interplanted onions have been shown to prevent aphid attack on adjacent potato plants by encouraging the production of terpenoids, a benefit exploited in the traditional practice of companion planting, while plants neighboring infested plants showed increased root growth at the expense of the extension of aerial parts.[32] The wild potato, Solanum berthaultii, produces an aphid alarm pheromone, (E)-β-farnesene, as an allomone, a pheromone to ward off attack; it effectively repels the aphid Myzus persicae at a range of up to 3 millimetres.[114] S. berthaultii and other wild potato species have a further anti-aphid defence in the form of glandular hairs which, when broken by aphids, discharge a sticky liquid that can immobilise some 30% of the aphids infesting a plant.[115]
Plants exhibiting aphid damage can have a variety of symptoms, such as decreased growth rates, mottled leaves, yellowing, stunted growth, curled leaves, browning, wilting, low yields, and death. The removal of sap creates a lack of vigor in the plant, and aphid saliva is toxic to plants. Aphids frequently transmit plant viruses to their hosts, such as to potatoes, cereals, sugarbeets, and citrus plants.[29] There are two types of virus transmission between plant-aphid interactions: non-circulative transmission and circulative transmission. In non-circulative transmission, the virus attaches itself to the aphids mouthparts and is released when the aphids feed on a different plant. These non-circulatory transmitted viruses promotes rapid dispersion of the vector, or aphids. In circulative transmission, the virus is ingested and passes through the gut lining to enter the hemolymph, where it is then circulated throughout the entire body. After reaching the salivary glands, the virus is then released into the saliva upon transmission sites in plants. Circulatory transmitted viruses allows for long-term feeding by the aphids and increases the chances of being infected with the virus.[116] The green peach aphid, Myzus persicae, is a vector for more than 110 plant viruses. Cotton aphids (Aphis gossypii) often infect sugarcane, papaya and peanuts with viruses.[21] In plants which produce the phytoestrogen coumestrol, such as alfalfa, damage by aphids is linked with higher concentrations of coumestrol.[117]

The coating of plants with honeydew can contribute to the spread of fungi which can damage plants.[118][119] Honeydew produced by aphids has been observed to reduce the effectiveness of fungicides as well.[120]
A hypothesis that insect feeding may improve plant fitness was floated in the mid-1970s by Owen and Wiegert. It was felt that the excess honeydew would nourish soil micro-organisms, including nitrogen fixers. In a nitrogen-poor environment, this could provide an advantage to an infested plant over an uninfested plant. However, this does not appear to be supported by observational evidence.[121]
Sociality
[edit]Some aphids show some of the traits of eusociality, joining insects such as ants, bees, and termites. However, there are differences between these sexual social insects and the clonal aphids, which are all descended from a single female parthenogenetically and share an identical genome. About fifty species of aphid, scattered among the closely related, host-alternating lineages Eriosomatinae and Hormaphidinae, have some type of defensive morph. These are gall-creating species, with the colony living and feeding inside a gall that they form in the host's tissues. Among the clonal population of these aphids, there may be several distinct morphs and this lays the foundation for a possible specialization of function, in this case, a defensive caste. The soldier morphs are mostly first and second instars with the third instar being involved in Eriosoma moriokense and only in Smythurodes betae are adult soldiers known. The hind legs of soldiers are clawed, heavily sclerotized and the stylets are robust making it possible to rupture and crush small predators.[122] The larval soldiers are altruistic individuals, unable to advance to breeding adults but acting permanently in the interests of the colony. Another requirement for the development of sociality is provided by the gall, a colonial home to be defended by the soldiers.[123]
The soldiers of gall-forming aphids also carry out the job of cleaning the gall. The honeydew secreted by the aphids is coated in a powdery wax to form "liquid marbles"[124] that the soldiers roll out of the gall through small orifices.[101] Aphids that form closed galls use the plant's vascular system for their plumbing: the inner surfaces of the galls are highly absorbent and wastes are absorbed and carried away by the plant.[101]
Interactions with humans
[edit]Pest status
[edit]About 5000 species of aphid have been described and of these, some 450 species have colonized food and fiber crops. As direct feeders on plant sap, they damage crops and reduce yields, but they have a greater impact by being vectors of plant viruses. The transmission of these viruses depends on the movements of aphids between different parts of a plant, between nearby plants, and further afield. In this respect, the probing behavior of an aphid tasting a host is more damaging than lengthy aphid feeding and reproduction by stay-put individuals. The movement of aphids influences the timing of virus epidemics.[125] They are major pests of greenhouse crops and species often encountered in greenhouses include: green peach aphid (Myzus persicae), cotton or melon aphid (Aphis gossypii), potato aphid (Macrosiphum euphorbiae), foxglove aphid (Aulacorthum solani) and chrysanthemum aphid (Macrosiphoniella sanborni) and others, which cause leaf yellowing, distorted leaves, and plant stunting; the excreted honeydew is a growing medium for a number of fungal pathogens including black sooty molds from the genera Capnodium, Fumago, and Scorias which then infect leaves and inhibit growth by reducing photosynthesis.[126]
Aphids, especially during large outbreaks, have been known to trigger allergic inhalant reactions in sensitive humans.[127]
Dispersal can be by walking or flight, appetitive dispersal, or by migration. Winged aphids are weak fliers, lose their wings after a few days and only fly by day. Dispersal by flight is affected by the impact, air currents, gravity, precipitation, and other factors, or dispersal may be accidental, caused by the movement of plant materials, animals, farm machinery, vehicles, or aircraft.[125]
Control
[edit]
Insecticide control of aphids is difficult, as they breed rapidly, so even small areas missed may enable the population to recover promptly. Aphids may occupy the undersides of leaves where spray misses them, while systemic insecticides do not move satisfactorily into flower petals. Finally, some aphid species are resistant to common insecticide classes including carbamates, organophosphates, and pyrethroids.[128]
For small backyard infestations, spraying plants thoroughly with a strong water jet every few days may be sufficient protection. An insecticidal soap solution can be an effective household remedy to control aphids, but it only kills aphids on contact and has no residual effect. Soap spray may damage plants, especially at higher concentrations or at temperatures above 32 °C (90 °F); some plant species are sensitive to soap sprays.[113][129][130]

Aphid populations can be sampled using yellow-pan or Moericke traps. These are yellow containers with water that attract aphids.[131] Aphids respond positively to green and their attraction to yellow may not be a true colour preference but related to brightness. Their visual receptors peak in sensitivity from 440 to 480 nm and are insensitive in the red region. Moericke found that aphids avoided landing on white coverings placed on soil and were repelled even more by shiny aluminium surfaces.[132] Integrated pest management of various species of aphids can be achieved using biological insecticides based on fungi such as Lecanicillium lecanii, Beauveria bassiana or Isaria fumosorosea.[133] Fungi are the main pathogens of aphids; Entomophthorales can quickly cut aphid numbers in nature.[134]
Aphids may also be controlled by the release of natural enemies, in particular lady beetles and parasitoid wasps. However, since adult lady beetles tend to fly away within 48 hours after release, without laying eggs, repeated applications of large numbers of lady beetles are needed to be effective. For example, one large, heavily infested rose bush may take two applications of 1500 beetles each.[113][135]
The ability to produce allomones such as farnesene to repel and disperse aphids and to attract their predators has been experimentally transferred to transgenic Arabidopsis thaliana plants using an Eβf synthase gene in the hope that the approach could protect transgenic crops.[136] Eβ farnesene has however found to be ineffective in crop situations although stabler synthetic forms help improve the effectiveness of control using fungal spores and insecticides through increased uptake caused by movements of aphids.[137]
In human culture
[edit]Aphids are familiar to farmers and gardeners, mainly as pests. Peter Marren and Richard Mabey record that Gilbert White described an invading "army" of black aphids that arrived in his village of Selborne, Hampshire, England, in August 1774 in "great clouds", covering every plant, while in the unusually hot summer of 1783, White found that honeydew was so abundant as to "deface and destroy the beauties of my garden", though he thought the aphids were consuming rather than producing it.[138]
Infestation of the Chinese sumac (Rhus chinensis) by Chinese sumac aphids (Schlechtendalia chinensis) can create "Chinese galls" which are valued as a commercial product. As "Galla Chinensis", they are used in traditional Chinese medicine to treat coughs, diarrhea, night sweats, dysentery and to stop intestinal and uterine bleeding. Chinese galls are also an important source of tannins.[29]
See also
[edit]Notes
[edit]- ^ The term "black fly" is also used for the Simuliidae, among them the vector of river blindness.
- ^ Dairying ants also milk mealybugs and other insects.
References
[edit]- ^ Piper, Ross (2007). Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals. Greenwood Press. pp. 6–9. ISBN 978-0-313-33922-6.
- ^ a b "aphid noun". Oxford Dictionaries. Retrieved 21 February 2025.
late 19th cent.: back-formation from aphides, plural of aphis modern Latin, from Greek, perhaps a misreading of koris 'bug' (interpreting the characters κoρ 'kor' as αϕ 'aph').
- ^ a b Żyła, Dagmara; Homan, Agnieszka; Wegierek, Piotr (2017). "Polyphyly of the extinct family Oviparosiphidae and its implications for inferring aphid evolution (Hemiptera, Sternorrhyncha)". PLOS ONE. 12 (4) e0174791. Bibcode:2017PLoSO..1274791Z. doi:10.1371/journal.pone.0174791. PMC 5405925. PMID 28445493.
- ^ Berry, R. E.; Taylor, L. R. (1968). "High-Altitude Migration of Aphids in Maritime and Continental Climates". Journal of Animal Ecology. 37 (3): 713–722. Bibcode:1968JAnEc..37..713B. doi:10.2307/3084. JSTOR 3084.
- ^ Isard, Scott A.; Irwin, Michael E.; Hollinger, Steven E. (1990-10-01). "Vertical Distribution of Aphids (Homoptera: Aphididae) in the Planetary Boundary Layer". Environmental Entomology. 19 (5): 1473–1484. doi:10.1093/ee/19.5.1473.
- ^ Hill, L. (2012). "The currant lettuce aphid, Nasonovia ribisnigri arrives in Tasmania: Part 1". Victorian Entomologist. 42 (2): 29–31.
- ^ Margaritopoulos, John T.; Kasprowicz, Louise; Malloch, Gaynor L.; Fenton, Brian (2009-05-11). "Tracking the global dispersal of a cosmopolitan insect pest, the peach potato aphid". BMC Ecology. 9 (1): 13. Bibcode:2009BMCE....9...13M. doi:10.1186/1472-6785-9-13. PMC 2687420. PMID 19432979.
- ^ Szwedo, J.; Nel, A. (2011). "The oldest aphid insect from the Middle Triassic of the Vosges, France". Acta Palaeontologica Polonica. 56 (4): 757–766. Bibcode:2011AcPaP..56..757S. doi:10.4202/app.2010.0034.
- ^ a b Capinera, John L. (2008). Encyclopedia of Entomology. Springer Science & Business Media. pp. 193–194. ISBN 978-1-4020-6242-1. Archived from the original on 2020-08-05. Retrieved 2018-02-04.
- ^ Johnson, Christine; Agosti, Donat; Delabie, Jocques H.; Dumpert, Klaus; Williams, D. J.; von Tschirnhaus, Michael; Macshwitz, Ulrich (2001). "Acropyga and Azteca ants (Hymenoptera: Formicidae) with scale insects (Sternorrhyncha: Coccoidea): 20 million years of intimate symbiosis" (PDF). American Museum Novitates (3335): 1–18. doi:10.1206/0003-0082(2001)335<0001:AAAAHF>2.0.CO;2. S2CID 55067700. Archived (PDF) from the original on 2012-09-27. Retrieved 2010-10-18.
- ^ Russell, Louise M. (1968). "Studies on Fossil Aphids" (PDF). Bulletin of the Entomological Society of America. 14 (2): 139–140. doi:10.1093/besa/14.2.139a. Retrieved 2018-02-04.
- ^ a b c d e f Dixon, A. F. G. (1998). Aphid Ecology (2nd ed.). Chapman and Hall. ISBN 978-0-412-74180-7. Archived from the original on 2013-02-18. Retrieved 2016-05-24.
- ^ Von Dohlen, Carol D.; Moran, Nancy A. (2000). "Molecular data support a rapid radiation of aphids in the Cretaceous and multiple origins of host alternation". Biological Journal of the Linnean Society. 71 (4): 689–717. Bibcode:2000BJLS...71..689D. doi:10.1111/j.1095-8312.2000.tb01286.x.
- ^ Chen, Rui; Favret, Colin; Jiang, Liyun; Wang, Zhe; Qiao, Gexia (29 September 2015). "An aphid lineage maintains a bark-feeding niche while switching to and diversifying on conifers". Cladistics. 32 (5): 555–572. doi:10.1111/cla.12141. PMID 34740301. S2CID 86517289.
- ^ Gullan, Penny J.; Martin, Jon H. (2009). "Sternorrhyncha". Encyclopedia of Insects (2nd ed.).
- ^ Rohdendorf, B. B., ed. (1991). Fundamentals of Paleontology. Volume 9. Arthropoda, Tracheata, Chelicerata. Smithsonian Institution and National Science Foundation. pp. 267–274.
- ^ Sorensen, J. T. (2009). "Aphids". In Resh, Vincent H.; Cardé, R. T. (eds.). Encyclopedia of Insects (2 ed.). Academic Press. pp. 27–31.
- ^ "Superfamily Aphidoidea, Latreille, 1802". Aphid: species file. Archived from the original on 6 November 2017. Retrieved 3 February 2018.
- ^ Blackman, R. L.; Eastrop, V. F. (1994). Aphids on the World's Trees. An Identification and Information Guide. Wallingford: CAB International. ISBN 978-0-85198-877-1.
- ^ Granett, Jeffrey; Walker, M. Andrew; Kocsis, Laszlo; Omer, Amir D. (2001). "Biology and management of grape phylloxera". Annual Review of Entomology. 46 (1): 387–412. Bibcode:2001AREnt..46..387G. doi:10.1146/annurev.ento.46.1.387. PMID 11112174.
- ^ a b c d e f McGavin, George C. (1993). Bugs of the World. Infobase Publishing. ISBN 978-0-8160-2737-8.
- ^ Favret, C.; Eades, D.C. (2020). Miller, G.L.; Qiao, G.; Sano, Masakazu; Stekolshchikov, A.V. (eds.). "Aphid Species File - Aphidomorpha". Université de Montréal. aphid.speciesfile.org. Montreal, Canada: Aphid Species File. Archived from the original on February 24, 2021. Retrieved December 13, 2020.
- ^ Papasotiropoulos, Vassilis; Tsiamis, Georgios; Papaioannou, Charikleia; Kilias, George (2013). "A molecular phylogenetic study of aphids (Hemiptera: Aphididae) based on mitochondrial DNA sequence analysis". Journal of Biological Research-Thessaloniki. 20: 1–13. Archived from the original on 2018-01-16. Retrieved 2018-01-15.
- ^ Kim, Hyojoong; Lee, Seunghwan; Jang, Yikweon (September 2011). Moreau, Corrie S. (ed.). "Macroevolutionary Patterns in the Aphidini Aphids (Hemiptera: Aphididae): Diversification, Host Association, and Biogeographic Origins". PLOS ONE. 6 (9) e24749. Bibcode:2011PLoSO...624749K. doi:10.1371/journal.pone.0024749. PMC 3174202. PMID 21935453.
- ^ Ortiz-Rivas, Benjamín; Martínez-Torres, David (2010). "Combination of molecular data support the existence of three main lineages in the phylogeny of aphids (Hemiptera: Aphididae) and the basal position of the subfamily Lachninae". Molecular Phylogenetics and Evolution. 55 (1): 305–317. Bibcode:2010MolPE..55..305O. doi:10.1016/j.ympev.2009.12.005. PMID 20004730.
- ^ Clark, Marta A; Moran, Nancy A.; Baumann, Paul; Wernegreen, Jennifer J. (2000). "Cospeciation Between Bacterial Endosymbionts (Buchnera) and a Recent Radiation of Aphids (Uroleucon) and Pitfalls of Testing for Phylogenetic Congruence". Evolution. 54 (2): 517–25. doi:10.1554/0014-3820(2000)054[0517:CBBEBA]2.0.CO;2. PMID 10937228.
- ^ Nováková, Eva; Hypša, Václav; Klein, Joanne; Foottit, Robert G; von Dohlen, Carol D.; Moran, Nancy A. (2013). "Reconstructing the phylogeny of aphids (Hemiptera: Aphididae) using DNA of the obligate symbiont Buchnera aphidicola" (PDF). Molecular Phylogenetics and Evolution. 68 (1): 42–54. Bibcode:2013MolPE..68...42N. doi:10.1016/j.ympev.2013.03.016. PMID 23542003. Archived (PDF) from the original on 2017-08-10. Retrieved 2018-04-27.
- ^ Chen, Rui; Wang, Zhe; Chen, Jing; Jiang, Li-Yun; Qiao, Ge-Xia (2017). "Insect-bacteria parallel evolution in multiple-co-obligate-aphid association: A case in Lachninae (Hemiptera: Aphididae)". Scientific Reports. 7 (1): 10204. Bibcode:2017NatSR...710204C. doi:10.1038/s41598-017-10761-9. PMC 5579299. PMID 28860659.
- ^ a b c d e f g h Stroyan, Henry G. (1997). "Aphid". McGraw-Hill Encyclopedia of Science and Technology (8th ed.). ISBN 978-0-07-911504-1.
- ^ Mutti, Navdeep S. (2006). Molecular Studies of the Salivary Glands of the Pea Aphid, Acyrthosiphon pisum (Harris) (PDF) (Ph.D. thesis). Kansas State University. Archived (PDF) from the original on 2012-02-27. Retrieved 2008-07-12.
- ^ Jing, X.; White, T. A.; Yang, X.; Douglas, A. E. (2015). "The molecular correlates of organ loss: the case of insect Malpighian tubules - PMC". Biology Letters. 11 (5). doi:10.1098/rsbl.2015.0154. PMC 4455741. PMID 25972400.
- ^ a b c van Emden, Helmut F.; Harrington, Richard (2017). Aphids as Crop Pests. CABI. pp. 189–190. ISBN 978-1-78064-709-8.
- ^ Powell, Glen; Tosh, Colin R.; Hardie, Jim (2005). "Host plant selection by aphids: Behavioral, Evolutionary, and Applied Perspectives". Annual Review of Entomology. 51 (1): 309–330. doi:10.1146/annurev.ento.51.110104.151107. PMID 16332214.
- ^ Spiller, N. J.; Koenders, L.; Tjallingii, W. F. (1990). "Xylem ingestion by aphids – a strategy for maintaining water balance". Entomologia Experimentalis et Applicata. 55 (2): 101–104. doi:10.1007/BF00352570 (inactive 11 July 2025).
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ a b Fisher, D. B. (2000). "Long distance transport". In Buchanan, Bob B.; Gruissem, Wilhelm; Jones, Russell L. (eds.). Biochemistry and Molecular Biology of Plants (4th ed.). Rockville, Maryland: American Society of Plant Physiologists. pp. 730–784. ISBN 978-0-943088-39-6.
- ^ Malone, M.; Watson, R.; Pritchard, J. (1999). "The spittlebug Philaenus spumarius feeds from mature xylem at the full hydraulic tension of the transpiration stream". New Phytologist. 143 (2): 261–271. Bibcode:1999NewPh.143..261M. doi:10.1046/j.1469-8137.1999.00448.x. JSTOR 2588576.
- ^ Powell, Glen; Hardie, Jim (2002). "Xylem ingestion by winged aphids". Entomologia Experimentalis et Applicata. 104 (1): 103–108. doi:10.1023/A:1021234412475 (inactive 12 July 2025).
{{cite journal}}: CS1 maint: DOI inactive as of July 2025 (link) - ^ a b c d Pompon, Julien; Quiring, Dan; Giordanengo, Philippe; Pelletier, Yvan (2010). "Role of xylem consumption on osmoregulation in Macrosiphum euphorbiae (Thomas)" (PDF). Journal of Insect Physiology. 56 (6): 610–615. Bibcode:2010JInsP..56..610P. doi:10.1016/j.jinsphys.2009.12.009. PMID 20036244. Archived from the original (PDF) on 16 July 2011.
- ^ Kingsolver, J. G.; Daniel, T. L. (1995). "Mechanics of Food Handling by Fluid-Feeding Insects". In Chapman, R. F.; de Boer, Gerrit (eds.). Regulatory mechanisms in insect feeding. Springer. pp. 60–65.
- ^ Ashford, D. A.; Smith, W. A.; Douglas, A. E. (2000). "Living on a high sugar diet: the fate of sucrose ingested by a phloem-feeding insect, the pea aphid Acyrthosiphon pisum". Journal of Insect Physiology. 46 (3): 335–341. Bibcode:2000JInsP..46..335A. doi:10.1016/S0022-1910(99)00186-9. PMID 12770238.
- ^ Wilkinson, T. L.; Ashfors, D. A.; Pritchard, J.; Douglas, A. E. (1997). "Honeydew sugars and osmoregulation in the pea aphid Acyrthosiphon pisum". Journal of Experimental Biology. 200 (11): 2137–2143. Bibcode:1997JExpB.200.2137W. doi:10.1242/jeb.200.15.2137. PMID 9320049. Archived from the original on 2008-08-21. Retrieved 2010-10-18.
- ^ Shakesby, A. J.; Wallace, I. S.; Isaacs, H. V.; Pritchard, J.; Roberts, D. M.; Douglas, A. E. (2009). "A water-specific aquaporin involved in aphid osmoregulation". Insect Biochemistry and Molecular Biology. 39 (1): 1–10. Bibcode:2009IBMB...39....1S. doi:10.1016/j.ibmb.2008.08.008. PMID 18983920.
- ^ Dadd, R. H.; Mittler, T. E. (1965). "Studies on the artificial feeding of the aphid Myzus persicae (Sulzer) – III. Some major nutritional requirements". Journal of Insect Physiology. 11 (6): 717–743. Bibcode:1965JInsP..11..717D. doi:10.1016/0022-1910(65)90154-X. PMID 5827534.
- ^ Buchner, Paul (1965). Endosymbiosis of animals with plant microorganisms. Interscience. ISBN 978-0-470-11517-6.
- ^ Whitehead, L. F.; Douglas, A. E. (1993). "A metabolic study of Buchnera, the intracellular bacterial symbionts of the pea aphid Acyrthosiphon pisum" (PDF). Journal of General Microbiology. 139 (4): 821–826. doi:10.1099/00221287-139-4-821.
- ^ Febvay, Gérard; Liadouze, Isabelle; Guillaud, Josette; Bonnot, Guy (1995). "Analysis of energetic amino acid metabolism in Acyrthosiphon pisum: a multidimensional approach to amino acid metabolism in aphids". Archives of Insect Biochemistry and Physiology. 29 (1): 45–69. doi:10.1002/arch.940290106.
- ^ Moran, Nancy A.; Jarvik, Tyler (2010). "Lateral transfer of genes from fungi underlies carotenoid production in aphids". Science. 328 (5978): 624–627. Bibcode:2010Sci...328..624M. doi:10.1126/science.1187113. PMID 20431015. S2CID 14785276.
- ^ Altincicek, B.; Kovacs, J.L.; Gerardo, N.M. (2012). "Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae". Biology Letters. 8 (2): 253–257. Bibcode:2012BiLet...8..253A. doi:10.1098/rsbl.2011.0704. PMC 3297373. PMID 21920958.
- ^ Valmalette, Jean Christophe; Dombrovsky, Aviv; Brat, Pierre; Mertz, Christian; Capovilla, Maria; Robichon, Alain (2012). "Light-induced electron transfer and ATP synthesis in a carotene synthesizing insect". Scientific Reports. 2 579. Bibcode:2012NatSR...2..579V. doi:10.1038/srep00579. PMC 3420219. PMID 22900140.
- ^ a b Wang, C. L.; Siang, L. Y.; Chang, G. S.; Chu, H. F. (1962). "Studies on the soybean aphid, Aphis glycines Matsumura". Acta Entomologica Sinica. 11: 31–44.
- ^ a b van Emden, Helmut F.; Harrington, Richard (2017). Aphids as Crop Pests, 2nd Edition. CABI. pp. 81–82. ISBN 978-1-78064-709-8. Archived from the original on 2021-06-24. Retrieved 2018-04-29.
- ^ Von Dohlen, Carol; Moran, Nancy A. (2000). "Molecular data support a rapid radiation of aphids in the Cretaceous and multiple origins of host alternation". Biological Journal of the Linnean Society. 71 (4): 689–717. Bibcode:2000BJLS...71..689V. doi:10.1006/bijl.2000.0470.
- ^ Moran, Nancy A. (1992). "The Evolution of Aphid Life Cycles". Annual Review of Entomology. 37: 321–348. doi:10.1146/annurev.en.37.010192.001541.
- ^ a b Blackman, Roger L. (2008). "Stability and variation in aphid clonal lineages". Biological Journal of the Linnean Society. 11 (3): 259–277. doi:10.1111/j.1095-8312.1979.tb00038.x.
- ^ a b Gullan, P. J.; Cranston, P. S. (2010). The Insects: An Outline of Entomology (4th ed.). Wiley. pp. 150–151. ISBN 978-1-118-84615-5.
- ^ a b c Hales, Dinah F.; Wilson, Alex C. C.; Sloane, Mathew A.; Simon, Jean-Christophe; Legallic, Jean-François; Sunnucks, Paul (2002). "Lack of Detectable Genetic Recombination on the X Chromosome During the Parthenogenetic Production of Female and Male Aphids". Genetics Research. 79 (3): 203–209. doi:10.1017/S0016672302005657. PMID 12220127. S2CID 25605477.
- ^ Nevo, Ettay; Coll, Moshe (2001). "Effect of nitrogen fertilization on Aphis gossypii (Homoptera: Aphididae): variation in size, color, and reproduction". Journal of Economic Entomology. 94 (1): 27–32. doi:10.1603/0022-0493-94.1.27. PMID 11233124. S2CID 25758038.
- ^ Jahn, Gary C.; Almazan, Liberty P.; Pacia, Jocelyn B. (2005). "Effect of nitrogen fertilizer on the intrinsic rate of increase of the rusty plum aphid, Hysteroneura setariae (Thomas) (Homoptera: Aphididae) on rice (Oryza sativa L.)" (PDF). Environmental Entomology. 34 (4): 938–943. doi:10.1603/0046-225X-34.4.938. S2CID 1941852. Archived from the original (PDF) on 2010-09-09.
- ^ Hughes, R. D. (1963). "Population Dynamics of the Cabbage Aphid, Brevicoryne brassicae (L.)". Journal of Animal Ecology. 32 (3): 393–424. Bibcode:1963JAnEc..32..393H. doi:10.2307/2600. JSTOR 2600.
- ^ Brisson, Jennifer A. (2010). "Aphid wing dimorphisms: linking environmental and genetic control of trait variation". Philosophical Transactions of the Royal Society B: Biological Sciences. 365 (1540): 605–616. doi:10.1098/rstb.2009.0255. PMC 2817143. PMID 20083636.
- ^ Weisser, Wolfgang W.; Zytynska, Sharon E.; Mehrparvar, Mohsen (2013-03-05). "Multiple Cues for Winged Morph Production in an Aphid Metacommunity". PLOS ONE. 8 (3) e58323. Bibcode:2013PLoSO...858323M. doi:10.1371/journal.pone.0058323. ISSN 1932-6203. PMC 3589340. PMID 23472179.
- ^ Lees, A. D. (1967-02-01). "The production of the apterous and alate forms in the aphid Megoura viciae Buckton, with special reference to the rôle of crowding". Journal of Insect Physiology. 13 (2): 289–318. Bibcode:1967JInsP..13..289L. doi:10.1016/0022-1910(67)90155-2. ISSN 0022-1910.
- ^ Kunert, Grit; Otto, Susanne; Röse, Ursula S. R.; Gershenzon, Jonathan; Weisser, Wolfgang W. (2005-04-28). "Alarm pheromone mediates production of winged dispersal morphs in aphids". Ecology Letters. 8 (6): 596–603. Bibcode:2005EcolL...8..596K. doi:10.1111/j.1461-0248.2005.00754.x. ISSN 1461-023X.
- ^ Ryabov, E. V.; Keane, G.; Naish, N.; Evered, C.; Winstanley, D. (2009-05-13). "Densovirus induces winged morphs in asexual clones of the rosy apple aphid, Dysaphis plantaginea". Proceedings of the National Academy of Sciences. 106 (21): 8465–8470. Bibcode:2009PNAS..106.8465R. doi:10.1073/pnas.0901389106. ISSN 0027-8424. PMC 2688996. PMID 19439653.
- ^ Chan, C. K. (1991). Aphid-transmitted viruses and their vectors of the world. Research Branch, Agriculture Canada. ISBN 0-662-18334-7. OCLC 872604083.
- ^ Reyes, Miguel L.; Laughton, Alice M.; Parker, Benjamin J.; Wichmann, Hannah; Fan, Maretta; Sok, Daniel; Hrček, Jan; Acevedo, Tarik; Gerardo, Nicole M. (2019-01-31). "The influence of symbiotic bacteria on reproductive strategies and wing polyphenism in pea aphids responding to stress". Journal of Animal Ecology. 88 (4): 601–611. Bibcode:2019JAnEc..88..601R. doi:10.1111/1365-2656.12942. ISSN 0021-8790. PMC 6453707. PMID 30629747.
- ^ Alford, David V. (2014). Pests of Fruit Crops: A Colour Handbook, Second Edition. CRC Press. pp. 71–72. ISBN 978-1-4822-5421-1. Archived from the original on 2021-06-24. Retrieved 2018-02-03.
- ^ a b Hooper-Bui, Linda M. (2008). "Ant". World Book Encyclopedia. ISBN 978-0-7166-0108-1.
- ^ Stadler, Bernhard; Dixon, Anthony F. G. (2005). "Ecology and Evolution of Aphid-Ant Interactions". Annual Review of Ecology, Evolution, and Systematics. 36 (1): 345–372. doi:10.1146/annurev.ecolsys.36.091704.175531.
- ^ Wootton, Anthony (1998). Insects of the World. Blandford. ISBN 978-0-7137-2366-3.
- ^ a b Neary, John (1977). Insects and Spiders. Time-Life Books. pp. 78–79. ISBN 978-0-8094-9687-7.
- ^ Salazar, Adrián; Fürstenau, Benjamin; Quero, Carmen; Pérez-Hidalgo, Nicolás; Carazo, Pau; Font, Enrique; Martínez-Torres, David (2015). "Aggressive mimicry coexists with mutualism in an aphid". Proceedings of the National Academy of Sciences. 112 (4): 1101–1106. Bibcode:2015PNAS..112.1101S. doi:10.1073/pnas.1414061112. PMC 4313836. PMID 25583474.
- ^ Baumann, Paul; Moran, Nancy A.; Baumann, Linda (2006). "Bacteriocyte-Associated Endosymbionts of Insects". In Dworkin, Martin; Falkow, Stanley; Rosenberg, Eugene; Schleifer, Karl-Heinz; Stackebrandt, Erko (eds.). The Prokaryotes. pp. 403–438. doi:10.1007/0-387-30741-9_16. ISBN 978-0-387-25476-0.
- ^ Douglas, A. E. (1998). "Nutritional Interactions in Insect-Microbial Symbioses: Aphids and Their Symbiotic BacteriaBuchnera". Annual Review of Entomology. 43 (1): 17–37. doi:10.1146/annurev.ento.43.1.17. PMID 15012383.
- ^ Bodył, Andrzej; Mackiewicz, Paweł; Gagat, Przemysław (2012). "Organelle Evolution: Paulinella Breaks a Paradigm". Current Biology. 22 (9): R304–R306. Bibcode:2012CBio...22.R304B. doi:10.1016/j.cub.2012.03.020. PMID 22575468.
- ^ Baumann, Linda; Baumann, Paul; Moran, Nancy A.; Sandström, Jonas; Thao, Mylo Ly (January 1999). "Genetic Characterization of Plasmids Containing Genes Encoding Enzymes of Leucine Biosynthesis in Endosymbionts (Buchnera) of Aphids". Journal of Molecular Evolution. 48 (1): 77–85. Bibcode:1999JMolE..48...77B. doi:10.1007/PL00006447. ISSN 0022-2844. PMID 9873079. S2CID 24062989. Archived from the original on 2021-11-13. Retrieved 2020-11-16.
- ^ Sabri, Ahmed; Vandermoten, Sophie; Leroy, Pascal D.; Haubruge, Eric; Hance, Thierry; Thonart, Philippe; De Pauw, Edwin; Francis, Frédéric (2013-09-25). "Proteomic Investigation of Aphid Honeydew Reveals an Unexpected Diversity of Proteins". PLOS ONE. 8 (9) e74656. Bibcode:2013PLoSO...874656S. doi:10.1371/journal.pone.0074656. ISSN 1932-6203. PMC 3783439. PMID 24086359.
- ^ Perez-Brocal, V.; Gil, R.; Ramos, S.; Lamelas, A.; Postigo, M.; Michelena, J.M.; Silva, F. J.; Moya, A.; Latorre, A. (2006). "A Small Microbial Genome: The End of a Long Symbiotic Relationship?". Science. 314 (5797): 312–313. Bibcode:2006Sci...314..312P. doi:10.1126/science.1130441. PMID 17038625. S2CID 40081627.
- ^ Mira, A.; Moran, Nancy A. (2002). "Estimating Population Size and Transmission Bottlenecks in Maternally Transmitted Endosymbiotic Bacteria". Microbial Ecology. 44 (2): 137–143. Bibcode:2002MicEc..44..137M. doi:10.1007/s00248-002-0012-9. PMID 12087426. S2CID 33681686.
- ^ Sakaki, Yoshiyuki; Shigenobu, Shuji; Watanabe, Hidemi; Hattori, Masahira; Ishikawa, Hajime (2000). "Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS". Nature. 407 (6800): 81–86. Bibcode:2000Natur.407...81S. doi:10.1038/35024074. PMID 10993077.
- ^ Viñuelas, José; Calevro, Federica; Remond, Didier; Bernillon, Jacques; Rahbé, Yvan; Febvay, Gérard; Fayard, Jean-Michel; Charles, Hubert (2007). "Conservation of the links between gene transcription and chromosomal organization in the highly reduced genome of Buchnera aphidicola". BMC Genomics. 8 (1): 143. doi:10.1186/1471-2164-8-143. PMC 1899503. PMID 17547756.
- ^ Moran, Nancy A.; Dunbar, Helen E.; Wilcox, Jennifer L. (2005). "Regulation of Transcription in a Reduced Bacterial Genome: Nutrient-Provisioning Genes of the Obligate Symbiont Buchnera aphidicola". Journal of Bacteriology. 187 (12): 4229–4237. Bibcode:2005JBact.187.4229M. doi:10.1128/JB.187.12.4229-4237.2005. PMC 1151715. PMID 15937185.
- ^ Tsuchida, T.; Koga, R.; Meng, X. Y.; T. Matsumoto; T. Fukatsu (2005). "Characterization of a facultative endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum". Microbial Ecology. 49 (1): 126–133. Bibcode:2005MicEc..49..126T. doi:10.1007/s00248-004-0216-2. PMID 15690225. S2CID 24144752.
- ^ Sakurai, M.; Koga, R.; Tsuchida, T.; Meng, X.-Y.; Fukatsu, T. (2005). "Rickettsia Symbiont in the Pea Aphid Acyrthosiphon pisum: Novel Cellular Tropism, Effect on Host Fitness, and Interaction with the Essential Symbiont Buchnera". Applied and Environmental Microbiology. 71 (7): 4069–4075. Bibcode:2005ApEnM..71.4069S. doi:10.1128/AEM.71.7.4069-4075.2005. PMC 1168972. PMID 16000822.
- ^ Ferrari, Julia; Scarborough, Claire L.; Godfray, H. Charles J. (2007). "Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids". Oecologia. 153 (2): 323–329. Bibcode:2007Oecol.153..323F. doi:10.1007/s00442-007-0730-2. PMID 17415589. S2CID 37052892.
- ^ Simon, J.-C.; Carre, S.; Boutin, M.; Prunier-Leterme, N.; Sabater-Munoz, B.; Latorre, A.; Bournoville, R. (2003). "Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts". Proceedings of the Royal Society B: Biological Sciences. 270 (1525): 1703–1712. doi:10.1098/rspb.2003.2430. PMC 1691435. PMID 12964998.
- ^ Weldon, Stephanie R.; Oliver, Kerry M. (2016). "Diverse Bacteriophage Roles in an Aphid-Bacterial Defensive Mutualism". The Mechanistic Benefits of Microbial Symbionts. Advances in Environmental Microbiology. Vol. 2. Springer, Cham. pp. 173–206. doi:10.1007/978-3-319-28068-4_7. ISBN 978-3-319-28066-0.
- ^ Weldon, S. R.; Strand, M. R.; Oliver, K. M. (2013-01-22). "Phage loss and the breakdown of a defensive symbiosis in aphids". Proceedings of the Royal Society of London B: Biological Sciences. 280 (1751) 20122103. Bibcode:2013PBioS.28022103W. doi:10.1098/rspb.2012.2103. PMC 3574403. PMID 23193123.
- ^ Oliver, K. M.; Moran, Nancy A.; Hunter, M. S. (2006). "Costs and benefits of a superinfection of facultative symbionts in aphids". Proceedings of the Royal Society B: Biological Sciences. 273 (1591): 1273–1280. doi:10.1098/rspb.2005.3436. PMC 1560284. PMID 16720402.
- ^ a b Capinera, John (2011). Insects and Wildlife: Arthropods and their Relationships with Wild Vertebrate Animals. John Wiley & Sons. p. 536. ISBN 978-1-4443-5784-4.
- ^ van Emden, Helmut F.; Harrington, Richard (2017). Aphids as Crop Pests. CABI. pp. 229–230. ISBN 978-1-78064-709-8.
- ^ a b c Brust, Gerald E. (22 June 2006). "Early season aphid and thrips populations". University of Maryland, College Park. Archived from the original on 19 July 2011. Retrieved 18 October 2010.
- ^ Lamb, K. P. (1961). "Some effects of fluctuating temperatures on metabolism, development, and rate of population growth in the cabbage aphid, Brevicoryne brassicae". Ecology. 42 (4): 740–745. Bibcode:1961Ecol...42..740L. doi:10.2307/1933502. JSTOR 1933502.
- ^ Jones, Margaret G. (1979). "Abundance of aphids on cereals from before 1973 to 1977". Journal of Applied Ecology. 16 (1): 1–22. Bibcode:1979JApEc..16....1J. doi:10.2307/2402724. JSTOR 2402724.
- ^ Krupke, Christian; Obermeyer, John; O'Neil, Robert (2007). "Soybean aphid, a new beginning for 2007". Pest and Crop. 7. Purdue University. Archived from the original on 2021-02-04. Retrieved 2008-07-17.
- ^ "Why some aphids can't stand the heat". Science Daily. 23 April 2007. Archived from the original on 15 January 2018. Retrieved 28 February 2018.
- ^ Hughes, R.D. (1963). "Population dynamics of the cabbage aphid, Brevicoryne brassicae (L.)". Journal of Animal Ecology. 32 (3): 393–424. Bibcode:1963JAnEc..32..393H. doi:10.2307/2600. JSTOR 2600.
- ^ Suwanbutr, S. (1996). "Stable age distributions of lucerne aphid populations in SE-Tasmania" (PDF). Thammasat International Journal of Science and Technology. 1 (5): 38–43. Archived from the original (PDF) on 10 September 2008.
- ^ Ostlie, Ken (3 August 2006). "Spider Mites, Aphids and Rain Complicating Spray Decisions in Soybean" (PDF). Minnesota Crop eNews. University of Minnesota. Archived from the original (PDF) on 10 September 2008.
- ^ Aoki, S. (1977). "Colophina clematis (Homoptera, Pemphigidae), an aphid species with soldiers" (PDF). Japanese Journal of Entomology. 45 (2): 276–282.[permanent dead link]
- ^ a b c Kutsukake, M.; Meng, X.Y.; Katayama, N.; Nikoh, N.; Shibao, H.; Fukatsu, T. (2012). "An insect-induced novel plant phenotype for sustaining social life in a closed system". Nature Communications. 3 1187: 1187–1193. Bibcode:2012NatCo...3.1187K. doi:10.1038/ncomms2187. PMC 3514493. PMID 23149732.
- ^ Preston-Mafham, Rod; Preston-Mafham, Ken (1993). The Encyclopedia of Land Invertebrate Behaviour. MIT Press. p. 281. ISBN 978-0-262-16137-4.
- ^ Gish, M.; Dafni, A.; Inbar, M. (2012). Heil, Martin (ed.). "Young Aphids Avoid Erroneous Dropping when Evading Mammalian Herbivores by Combining Input from Two Sensory Modalities". PLOS ONE. 7 (4) e32706. Bibcode:2012PLoSO...732706G. doi:10.1371/journal.pone.0032706. PMC 3322135. PMID 22496734.
- ^ Kazana, Eleanna; Pope, Tom W.; Tibbles, Laurienne; Bridges, Matthew; Pickett, John A.; Bones, Atle M.; Powell, Glen; Rossiter, John T. (2007). "The cabbage aphid: a walking mustard oil bomb". Proceedings of the Royal Society B. 274 (1623): 2271–2277. doi:10.1098/rspb.2007.0237. PMC 2288485. PMID 17623639.
- ^ Vilcinskas, Andreas (2016). "Aphid Immunity.". Biology and Ecology of Aphids. CRC Press. p. 131.
- ^ Edwards, John S. (1966). "Defence by smear: supercooling in the cornicle wax of aphids". Nature. 211 (5044): 73–74. Bibcode:1966Natur.211...73E. doi:10.1038/211073a0. S2CID 4295930.
- ^ Martinson, Candace (2008). "Aphid". World Book Encyclopedia. ISBN 978-0-7166-0108-1.
- ^ Way, M.J. (1963). "Mutualism between ants and honeydew-producing Homoptera". Annual Review of Entomology. 8: 307–344. doi:10.1146/annurev.en.08.010163.001515.
- ^ Grasswitz, Tessa R.; Paine, Timothy D. (1992). "Kairomonal effect of an aphid cornicle secretion on Lysiphlebus testaceipes (Cresson) (Hymenoptera: Aphidiidae)". Journal of Insect Behavior. 5 (4): 447–457. Bibcode:1992JIBeh...5..447G. doi:10.1007/BF01058190. S2CID 25298742.
- ^ Lenhart, Paul A.; Jackson, Kelly A.; White, Jennifer A. (2018). "Heritable variation in prey defence provides refuge for subdominant predators". Proceedings of the Royal Society B: Biological Sciences. 285 (1879) 20180523. doi:10.1098/rspb.2018.0523. PMC 5998095. PMID 29848647.
- ^ "Aphid Parasitoids". IET Department of the College of Agriculture and Natural Resources. extension.umd.edu. College Park, Maryland: University of Maryland. Archived from the original on 2018-06-26. Retrieved 2018-04-29 – via College of Agriculture & Natural Sciences.
Aphid parasitoids are very tiny wasps, about 1/10 inch long. They are slender, black or brown, and have a pinched or "wasp waist"
- ^ Havelka, Jan; Tomanović, Željko; Kavallieratos, Nickolas G.; Rakhshani, Ehsan; Pons, Xavier; Petrović, Andjeljko; Pike, Keith S.; Starý, Petr (2012-05-01). "Review and Key to the World Parasitoids (Hymenoptera: Braconidae: Aphidiinae) of Aphis ruborum (Hemiptera: Aphididae) and Its Role as a Host Reservoir". Annals of the Entomological Society of America. 105 (3): 386–394. doi:10.1603/an11108. S2CID 84348019.
- ^ a b c Flint, M.L. (July 2013). "Aphids". UC IPM. Archived from the original on 2018-04-09. Retrieved 6 February 2018.
- ^ Gibson, R. W.; Pickett, J. A. (1983). "Wild potato repels aphids by release of aphid alarm pheromone". Nature. 302 (5909): 608–609. Bibcode:1983Natur.302..608G. doi:10.1038/302608a0. S2CID 4345998.
- ^ Gibson, R. W. (1971). "Glandular hairs providing resistance to aphids in certain wild potato species". Annals of Applied Biology. 68 (2): 113–119. doi:10.1111/j.1744-7348.1971.tb06448.x.
- ^ Nalam, Vamsi (2019). "Plant defense against aphids, the pest extraordinaire". Plant Science. 279: 96–107. Bibcode:2019PlnSc.279...96N. doi:10.1016/j.plantsci.2018.04.027. PMID 30709498.
- ^ United States Department of Agriculture (2003). Studies on the Chemical and Biological Properties of Coumestrol and Related Compounds. US Government Printing Office. pp. 47–67. Archived from the original on 2015-04-02. Retrieved 2015-04-26.
- ^ Gillman, Daniel H. (2005). "Sooty mold" (PDF). University of Massachusetts Amherst. Archived (PDF) from the original on September 28, 2011. Retrieved October 18, 2010.
- ^ Reynolds, Hannah T.; Volk, Tom (September 2007). "Scorias spongiosa, the beech aphid poop-eater". Tom Volk's Fungus of the Month. University of Wisconsin–La Crosse. Archived from the original on July 30, 2010. Retrieved October 18, 2010.
- ^ Dik, A. J.; van Pelt, J. A. (1992). "Interaction between phyllosphere yeasts, aphid honeydew and fungicide effectiveness in wheat under field conditions". Plant Pathology. 41 (6): 661–675. Bibcode:1992PPath..41..661D. doi:10.1111/j.1365-3059.1992.tb02550.x.
- ^ Choudhury, Dhrupad (1985). "Aphid honeydew: a re-appraisal of the hypothesis of Owen and Wiegert". Oikos. 45 (2): 287–290. doi:10.2307/3565718. JSTOR 3565718.
- ^ Stern, D. L.; Foster, W. A. (1996). "The evolution of soldiers in aphids". Biological Reviews of the Cambridge Philosophical Society. 71 (1): 27–79. doi:10.1111/j.1469-185X.1996.tb00741.x. PMID 8603120. S2CID 8991755.
- ^ Choe, Jae C.; Crespi, Bernard J. (1997). The Evolution of Social Behaviour in Insects and Arachnids. Cambridge University Press. pp. 150–152. ISBN 978-0-521-58977-2.
- ^ Pike, N.; Richard, D.; Foster, W.; Mahadevan, L. (2002). "How aphids lose their marbles". Proceedings of the Royal Society B: Biological Sciences. 269 (1497): 1211–5. Bibcode:2002PBioS.269.1211P. doi:10.1098/rspb.2002.1999. PMC 1691028. PMID 12065036.
- ^ a b van Emden, Helmut F.; Harrington, Richard (2017). Aphids as Crop Pests. CABI. pp. 196–198. ISBN 978-1-78064-709-8.
- ^ Cloyd, Raymond (14 February 2022). "Aphids". Greenhouse Product News. 32: 14.
- ^ Shulman, Sidney (1967-01-01). "Allergic Responses to Insects". Annual Review of Entomology. 12 (1): 323–346. doi:10.1146/annurev.en.12.010167.001543.
- ^ Pundt, Leanne (2011). "Managing Aphids in the Greenhouse". University of Connecticut College of Agriculture, Health, and Natural Resources. Archived from the original on 2018-02-18. Retrieved 20 February 2018.
- ^ Cranshaw, W.S. (March 2008). "Insect Control: Soaps and Detergents". University of Colorado. Archived from the original on 7 February 2018. Retrieved 6 February 2018.
- ^ Ubl, J.B. (July 2009). "Insecticidal Soaps for Garden Pest Control". Clemson University. Archived from the original on 22 January 2018. Retrieved 6 February 2018.
- ^ Evans, D. A.; Medler, J. T. (1966-12-01). "Improved Method of Using Yellow-Pan Aphid Traps1". Journal of Economic Entomology. 59 (6): 1526–1527. doi:10.1093/jee/59.6.1526. ISSN 1938-291X. Archived from the original on 2021-11-13. Retrieved 2020-03-22.
- ^ Döring, Thomas Felix; Chittka, Lars (2007). "Visual ecology of aphids—a critical review on the role of colours in host finding" (PDF). Arthropod-Plant Interactions. 1 (1): 3–16. Bibcode:2007APInt...1....3D. doi:10.1007/s11829-006-9000-1. S2CID 20066025. Archived (PDF) from the original on 2020-09-19. Retrieved 2020-08-27.
- ^ Jaronski, S. T.; Mascarin, G. M. (2017). "Mass Production of Fungal Entomopathogens". In Lacey, Lawrence (ed.). Microbial control of insect and mite pests: from theory to practice. Academic Press. pp. 141–155. ISBN 978-0-12-803527-6.
- ^ Steinkraus, Donald C. (2006). "Factors affecting transmission of fungal pathogens of aphids". Journal of Invertebrate Pathology. 92 (3): 125–131. Bibcode:2006JInvP..92..125S. doi:10.1016/j.jip.2006.03.009. PMID 16780867.
- ^ Lady Beetle Releases for Aphid Control: How to Help Them Work Archived 2015-04-02 at the Wayback Machine. Clark, J.K., University of California Davis, June 2011.
- ^ Beale, M. H.; Birkett, M. A.; Bruce, T. J. A.; Chamberlain, K.; Field, L. M.; Huttly, A. K.; Martin, J. L.; Parker, R.; Phillips, A. L.; Pickett, J. A.; Prosser, I. M.; Shewry, P. R.; Smart, L. E.; Wadhams, L. J.; Woodcock, C. M.; Zhang, Y. (2006). "Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior". Proceedings of the National Academy of Sciences. 103 (27): 10509–10513. Bibcode:2006PNAS..10310509B. doi:10.1073/pnas.0603998103. PMC 1502488. PMID 16798877.
- ^ Pickett, J. A.; Wadhams, L. J.; Woodcock, C. M.; Hardie, J. (1992). "The Chemical Ecology of Aphids". Annual Review of Entomology. 37 (1): 67–90. doi:10.1146/annurev.en.37.010192.000435.
- ^ Marren, Peter; Mabey, Richard (2010). Bugs Britannica. Chatto & Windus. pp. 191–194. ISBN 978-0-7011-8180-2. Archived from the original on 2017-03-14. Retrieved 2018-02-09.
External links
[edit]- Aphids of southeastern U.S. woody ornamentals
- Acyrthosiphon pisum, MetaPathogen – facts, life cycle, life cycle image
- Sequenced Genome of Pea Aphid, Agricultural Research Service
- Insect Olfaction of Plant Odour: Colorado Potato Beetle and Aphid Studies
- Asian woolly hackberry aphid, Center for Invasive Species Research
On the University of Florida / Institute of Food and Agricultural Sciences Featured Creatures website:
- Aphis gossypii, melon or cotton aphid
- Aphis nerii, oleander aphid
- Hyadaphis coriandri, coriander aphid
- Longistigma caryae, giant bark aphid
- Myzus persicae, green peach aphid
- Sarucallis kahawaluokalani, crapemyrtle aphid
- Shivaphis celti, an Asian woolly hackberry aphid
- Toxoptera citricida, brown citrus aphid


















